Health Matters is a biweekly opinion column. The views expressed are solely the author’s.
Health Matters is a biweekly opinion column. The views expressed are solely the author’s.
The short answer is an emphatic yes. 100%. Absolutely.
The question has evolved into when to vaccinate after COVID-19 symptoms resolve (recommendation is 90 days) and if a second shot is even necessary if you are a COVID survivor.
Contracting COVID-19 confers natural immunity for an estimated five months at an 83% risk reduction of infection, but those numbers are highly variable depending on the severity of disease. For example, if only mild symptoms occurred during the COVID infection, then less antibodies were made and thus shorter immunity effectiveness and duration compared to the patient with more severe symptoms.
The vaccine offers superior and more predictable protection, and evens the playing field so that all recipients have a baseline expected antibody level. For those wondering, we do not know how long the vaccines provide protection, but we do know it is longer than what natural immunity offers.
So we have established that if you had COVID-19 you should get the vaccine. The next question is if a second shot is needed. A few studies published in recent weeks have suggested that one shot may build up the immune system sufficiently, even superfluously, if COVID was previously contracted.
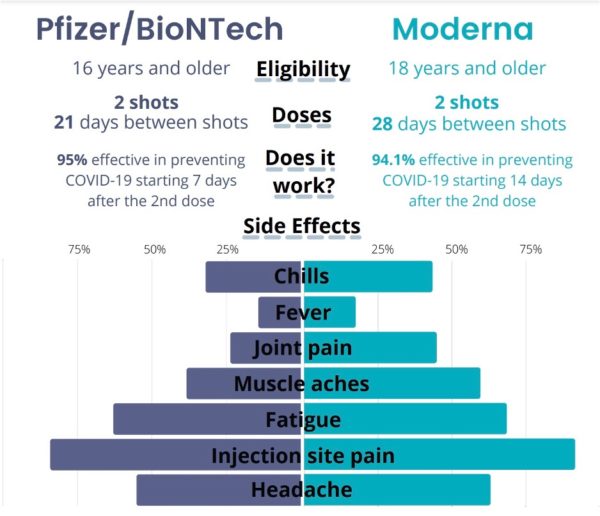
One trend researchers have noticed is more reactogenicity — fatigue, headache, chills, fevers and muscle pain — after the first shot for COVID survivors compared to COVID-naive recipients. One recent yet-to-be peer reviewed study tries to explain this phenomenon, revealing that the antibody response to the first shot was equal to or greater than the antibody response to the second shot in COVID-naive recipients. This was also reflected in the significantly higher reactogenicity in individuals who had been infected.
Another preliminary study echoed the findings of higher antibody count after the first shot for COVID survivors. This research has led some scientists to suggest a more evidence-based approach to vaccine protocol and distribution:
- Only one shot is needed if previously COVID positive
- Patients who have had laboratory-confirmed COVID-19 can be placed lower on the vaccination priority list. This in effect would increase vaccine availability to others — especially in areas of vaccine shortage — and avoid unnecessary painful side effects of a second shot.
While an evidence-based approach seems logical, modifying an FDA-approved vaccine schedule can be precarious. First, some patients with mild cases may not get the robust antibody spike on first shot like the others, so a second shot may still be necessary to achieve immunity. Second, a COVID survivor may not have antibodies for the more contagious variants, which vaccines have effectiveness against based on some early studies.
My take would be to follow the schedule of two doses regardless of COVID history, at least for now. Making exceptions for the second shot seems too risky at this early stage of the vaccine rollout. Moreover, the studies approved by the FDA were performed with two shots, and no studies skipping the second dose have been peer-reviewed or large scale (the studies mentioned above were between 30-100 patients — hardly large scale). Lastly, there is no known harm from taking a second shot if previously COVID positive.
To date, Arlington County has a reported 13,729 cases of known COVID-19, which is the lowest incidence among neighboring counties adjusted for population (Arlington: 5,780, Alexandria: 6,629 and Fairfax: 6,000 cases per 100,000). It is not clear how many of these Arlington COVID survivors have gotten the vaccine, but this large number underscores the importance of spreading the word that if you had COVID, a vaccine is still recommended.
Dr. George C. Hwang, known to his patients as Dr. Chaucer, is a practicing anesthesiologist who also helps to run Mind Peace Clinics in Arlington. He has written for multiple journals, textbooks and medical news outlets, and has been living in Arlington for the past 15 years.